Scientists Uncover the Secrets of an 80-Year-Old Rare-Earth Element
Scientists have made a groundbreaking discovery, unraveling the mysteries of the rare Earth mineral Promethium, which had previously eluded researchers for the past 80 years since its discovery.
Researchers from the Oak Ridge National Laboratory utilized a new technique that allowed them to create a pure isotope of this unique lanthanum element. This significant breakthrough has illuminated the element’s properties, sparking a wave of excitement in the scientific community.
The Chemical World
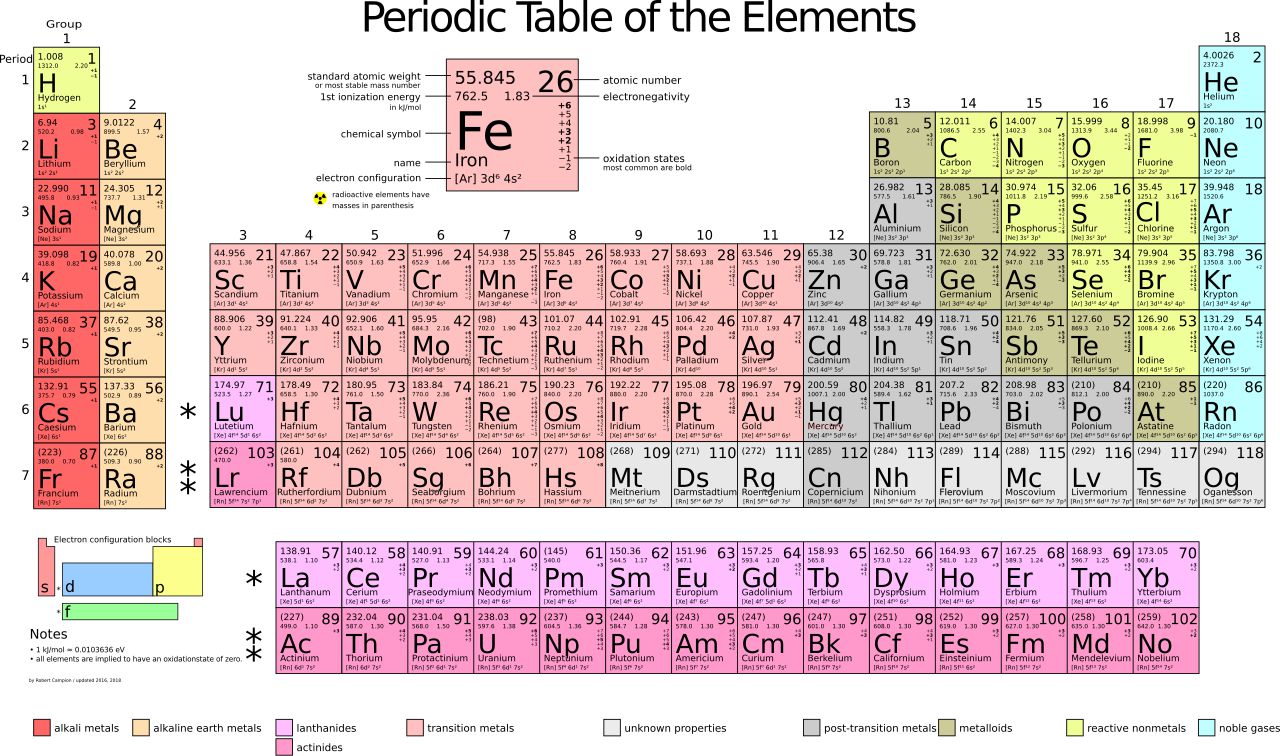
The Periodic Table of Elements stands as a testimony to the strides scientists and researchers have made in understanding the chemical composition of our world.
Source: Wikimedia
Despite our incredible knowledge of the elements on the table, some we know more about than others, and the properties of a few, such as promethium, have left scientists scratching their heads for decades.
A Tour de Force
Published in Nature, the study’s findings fill a massive gap in chemistry textbooks, and could eventually lead to better methods for separating promethium from similar elements in nuclear waste.

Source: Freepik
“It’s a tour de force,” says Polly Arnold, a chemist at Lawrence Berkeley National Laboratory in Berkeley, California, who was not involved in the research.
We Know Little About This Rare-Earth Element
Researchers estimate that less than 1 kilogram of promethium currently exists in the Earth’s crust, and scientists have previously harnessed its radiation to power pacemakers and spacecraft.

National Archives at College Park/Wikimedia Commons
Although Earth’s crust contains many rare-earth elements, they are thinly spread and difficult to isolate due to their very similar chemistry. This makes it challenging to extract just one lanthanide element and isolate it from the rest.
The Mystery of Promethium
Promethium was first discovered back in 1945 and was placed into the lanthanide elemental category, which comprises 15 metallic chemicals often dubbed rare earth metals.

Source: Wikimedia
Its atomic number is 61, which refers to the number of protons in its nucleus. For over 80 years, we knew very little about this element, and most of its chemical properties remained a mystery to researchers.
Scientists Can Utilize Promethium Despite Its Mysteries
Despite promethium’s mysterious nature, scientists were able to determine some of its properties, including strong magnetism, which allowed them to use the element in several modern products, including atomic batteries and smartphones.

Source: Wikimedia
“They are used in lasers; they are part of the screens of your smartphone. They are also used in very strong magnets in wind turbines and electric vehicles,” said Ilja Popovs, a research and development staff member at Oak Ridge National Laboratory (ORNL) and
Element Continues to Evade Researchers
The study of Promethium has presented formidable challenges, primarily due to its inherently unstable and radioactive nature. This characteristic often leads to its transformation into other elements before researchers can conduct thorough examinations.

Source: Freepik
“Promethium doesn’t have a stable isotope — they’re all radioactive, meaning that they are decaying [into other elements] with time,” Ivanov said. “You get this element through a fission process, so it’s scarce and difficult to study.”
The Creation of Promethium 147
Scientists working at the Oak Ridge National Laboratory, which coincidentally is the precursor to the original lab that discovered Promethium, implemented a new process that permitted them to create an isotope of Promethium called Promethium-147.

Source: Freepik
Following this, the researchers combined Promethium-147 with a ligand, which allowed them to create a stable isotope that could be examined in water.
Understanding the Rare Element
“The whole idea was to explore this very rare element to gain new knowledge,” said Alex Ivanov, an ORNL scientist who co-led the research, (via Phys).

Source: Christina @ wocintechchat.com/Unsplash
“Once we realized it was discovered at this national lab and the place where we work, we felt an obligation to conduct this research to uphold the ORNL legacy.”
Researchers Share the Results of the Study in a Journal
For the first time, scientists were able to conduct tests of the bonding properties of Promethium using X-ray spectroscopy, and the results of their work were shared in the journal Nature.

Source: Wikimedia
While happy with their results, the researchers explained it was no easy task. “Because promethium is radioactive, once it’s decaying, it’s getting transmuted into the adjacent element, which is samarium,” Ivanov said. “So you will have a tiny amount of contamination in the form of samarium.”
Filling in the Gap
“There are thousands of publications on lanthanides’ chemistry without promethium. That was a glaring gap for all of science,” said ORNL’s Santa Jansone-Popova, who co-led the study.

Source: Polina Tankilevitch/Pexels
“Scientists have to assume most of its properties. Now we can actually measure some of them,” Jansone-Popova notes.
Kicking Off the Study
The ORNL-led team of scientists kicked off the research by preparing a chemical complex of promethium, which allowed the element to show off its characterization in solution for the first time.

Source: Wikimedia
Doing this allowed the researchers to look at the secrets of this extremely rare lanthanide.
The Last Piece of the Puzzle
The study details how the researchers bombarded the promethium using a highly technical process that utilized high-energy photons enhanced by a particle accelerator.

Source: Wikimedia
This allowed the scientists to gain insight into the positions of atoms and the lengths of bonds that comprise the element.
Pulling the Element Apart
According to Scientific American, current separation methods that often use molecules bind positively charged lanthanide ions in solution, forming coordination complexes.

Source: Wikimedia
Chemists can then exploit the differences between these complexes to separate them. “But you need lots and lots of repeated separations to get to the pure material,” says Oak Ridge chemist Ilja Popovs, who co-led the research.
Comparison With Other Elements
Essentially, the experiment’s results allowed researchers to compare promethium’s properties with those of other rare earth metals for the first time since its discovery.

Source: Wikimedia
“Because it has no stable isotopes, promethium was the last lanthanide to be discovered and has been the most difficult to study,” said ORNL’s Popovs in a press statement.
Adding the Missing Link
According to the study’s co-author, the analysis of promethium will allow researchers to better understand rare Earth metals as a whole.

Source: Freepik
“Anything that we would call a modern marvel of technology would include, in one shape or another, these rare earth elements; we are adding the missing link,” said Popovs.
Defining Promethium’s Shape
The team provided the first demonstration of a lanthanide contraction feature, which showed that the radii of their ions decrease as the atomic numbers increase. This contraction creates chemical and electronic properties by confining the same charge to a shrinking space.

Source: Wikimedia
The scientists behind the study got a clear understanding of the promethium signal, which helped them define the shape of the trend.
Making Supercomputer-Worthy Calculations
The team also performed quantum chemical calculations and molecular dynamics simulations at the Oak Ridge Leadership Computing Facility using the only supercomputer capable of calculating computational resources.

Source: Sora Shimazaki/Pexels
The researchers expect that future calculations will take place on ORNL’s Frontier, the world’s most powerful supercomputer and the first exascale system.
A Landmark Moment
“It’s really astonishing from a scientific viewpoint. I was struck once we had all the data,” said Ivanov. “The contraction of this chemical bond accelerates along this atomic series, but after promethium, it considerably slows down.”

Source: Cytonn Photography/Pexels
Ivanov continued: This is an important landmark in understanding the chemical bonding properties of these elements and their structural changes along the periodic table.”
Increased Production of Promethium
With the newfound ability to study the bonding properties of Promethium, researchers from ORNL may be able to produce larger quantities of this rare metal, which could potentially revolutionize the production of modern devices.

Source: Freepik
According to the researchers, it may also allow them to separate the element from other lanthanides.
Successfully Separating Promethium From Lanthanides
“You cannot utilize all these lanthanides as a mixture in modern advanced technologies because first, you need to separate them,” said Santa Jansone-Popova,

Source: Wikimedia
They continued, “This is where the contraction becomes very important; it basically allows us to separate them, which is still quite a difficult task.”
Other Unknown Elements
We know very little about a handful of elements on the periodic table beyond their existence. Scientists have largely identified Element 120, or Unbinilium, as a “hypothetical chemical element,” which, once discovered, will be the heaviest element on the planet.
Source: Wikimedia Commons
Exercise submitted their findings in a paper titled “Heaviest Element Yet Within Reach After Breakthrough,” which the Nature Journal published.
The Groundbreaking Research
Hiromitsu Haba, who leads the Superheavy Element Research Group at RIKEN Nishima Center for Accelerator-based Science in Saitama, Japan said, “The search for the superheavy elements beyond 118, oganesson, is proving to be a great challenge.”

Source: chokniti/Freepik
“Data from the experiment will greatly improve the accuracy of existing theoretical calculations, and will greatly advance mankind towards the discovery of elements 119 and 120,” Haba notes.
Creating a New Element
Jacklyn Gates, a nuclear scientist at the California lab who is leading the effort, said, “We had never demonstrated this reaction before, and proving it was possible was essential before we embarked on our attempt to make 120.”

Source: Rodolfo Clix/Wikimedia Commons
Gates continues: “The creation of a new element is an extremely rare feat. It’s exciting to be a part of the process and to have a promising path forward.”
